.jpg)
POCT fluorescence platform
【Intended use】
【Principal component】
1.Reagent kit included in the kit
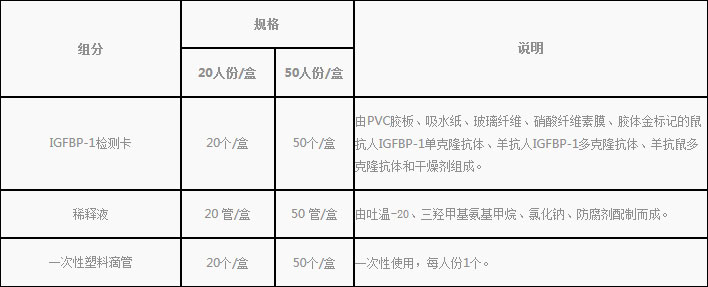
2.Products do not contain, need to bring their own supplies
Disposable sterile vaginal swab
【Storage conditions and validity】
Reagent kit should be in 4 degrees Celsius to 30 degrees Celsius conditions to avoid light, dry storage, not frozen. The product is valid for 12 months. After opening the seal, the reagent card should be used within 1 hours as soon as possible. See box or label production date
【Suitable instrument】
nothing
【Sample requirement】
1.Sample type: vaginal discharge.
2.Sample collection: disposable vaginal swab into vaginal fornix posterior or at the cervix to stop after 5 seconds the cotton swabs of vaginal secretions, the swab is put in dilute solution, turn the swab 10 to 20 seconds, the vaginal swab on the fully dissolved in diluted liquid. Samples have been collected in a timely manner to detect the best effect.
3.The samples are contaminated by blood, lubricant, disinfectant, etc., which may interfere with the test results.
【Test method】
1.Before using this product, please check all the items in the box. Before preparing the test, please do not tear the aluminum foil bag.
2.The reagent box inner foil bag and the dilution liquid are taken out at room temperature (15 degrees C to 30 C). Must be used before the dilution bottle two, to ensure that all of the liquid falls on the bottom of the diluted liquid bottle.
3.The samples were collected by the swab samples, and the samples were collected and the samples were collected in the diluted solution.
4.Open the aluminum foil bag, take out the test paper strip. Remove the detection card from the aluminum foil bag, should be used as soon as possible within 1 hours.
5.Remove the disposable plastic dropper from the kit, draw sample handling liquid enough from dilute solution, the vertical downward drop into the amount (about 3 drops) sample adding hole sample treatment solution to the detection card.
6.Place the test card on a clean, dry and flat surface, and read the results within 15 minutes.
【Positive judgment】
Positive judgment value: should not be less than 25 ng/mL.
Through 100 cases of clinical samples (47 cases of positive samples, 53 cases of negative samples) for IGFBP-1 detection and statistical analysis, the positive judgment.
Test results only reaction samples at the time of the state, for reference only. Diagnosis should be combined with clinical symptoms and other experimental evaluation, it is recommended to establish their own laboratory reference interval.
【Interpretation of test results】
1.Positive: in the detection area (T) and control area (C) each appeared a ribbon, the test results were positive.
2.Negative: only in the control area (C) appears a ribbon, the test results were negative.
3.Invalid: control area (C) no ribbon appears, indicating that the test is not valid, should be re tested.
【Limitations of test methods】
1.This product is a kind of qualitative detection, can not get quantitative results.
2.The interpretation of the results must be combined with clinical and therapeutic background.
3.If more obvious bleeding occurs (swab side obviously become red), should be paid attention to read the results.
4.In a very small number of cases, when the rupture of membranes after more than 12 hours after the extraction of samples, due to the impact of vaginal protease caused IGFBP-1 degradation and produce false negative results.
5.Vaginal disinfectant lotion or vaginal drugs may lead to false negative results, should be stopped using 6 hours before the test.
【Product performance index】
1.Sensitivity: the minimum detectable amount of insulin-like growth factor binding protein -1 was 25ng/ml.
2.The specificity and the concentration of 100ng/ml (Human Insulin), and insulin concentrations of gonadotropin human chorionic 100IU/l (Human Chorionic Gonadotropin, HCG) were measured. The test results should be negative, no cross reaction.
【Matters needing attention】
1.Only for in vitro diagnosis, the product should be read in detail before using the specification, in the period of validity.
2.In the process of specimen collection and testing, should do a good job of aseptic operation and personal safety protection; the use of the reagent immediately after the destruction, with the hospital or environmental protection departments should be required to dispose of waste.
3.This product is only outside the donor diagnosis, disposable, packaging damage should not be used, test strips, dilution, and so can not be reused.
4.Do not use expired, damaged or contaminated reagents.
【Identity interpretation】
Graphical symbols for use in reagent kits
Production license number:粵食藥監(jiān)械生產(chǎn)許20122276號(hào)
【醫(yī)療器械注冊(cè)證編號(hào)/產(chǎn)品技術(shù)要求編號(hào)】粵械注準(zhǔn)20162400007
